
(The Hill) – Lupin Pharmaceuticals Inc. recalls four lots of its blood pressure medication, Quinapril Tablets, because of an impurity known as Nitrosamines being found in recent testing of the product, according to the Food and Drug Administration (FDA).
The FDA stated that no illness related to the medication has yet been reported and that the marketing of the Quinapril Tablets ended in September. Quinapril is an angiotensin-converting enzyme (ACE) inhibitor used to treat hypertension and lower blood pressure.
The tablets were contaminated by a substance known as Nitrosamines, which the FDA reports is commonly found in food and water.





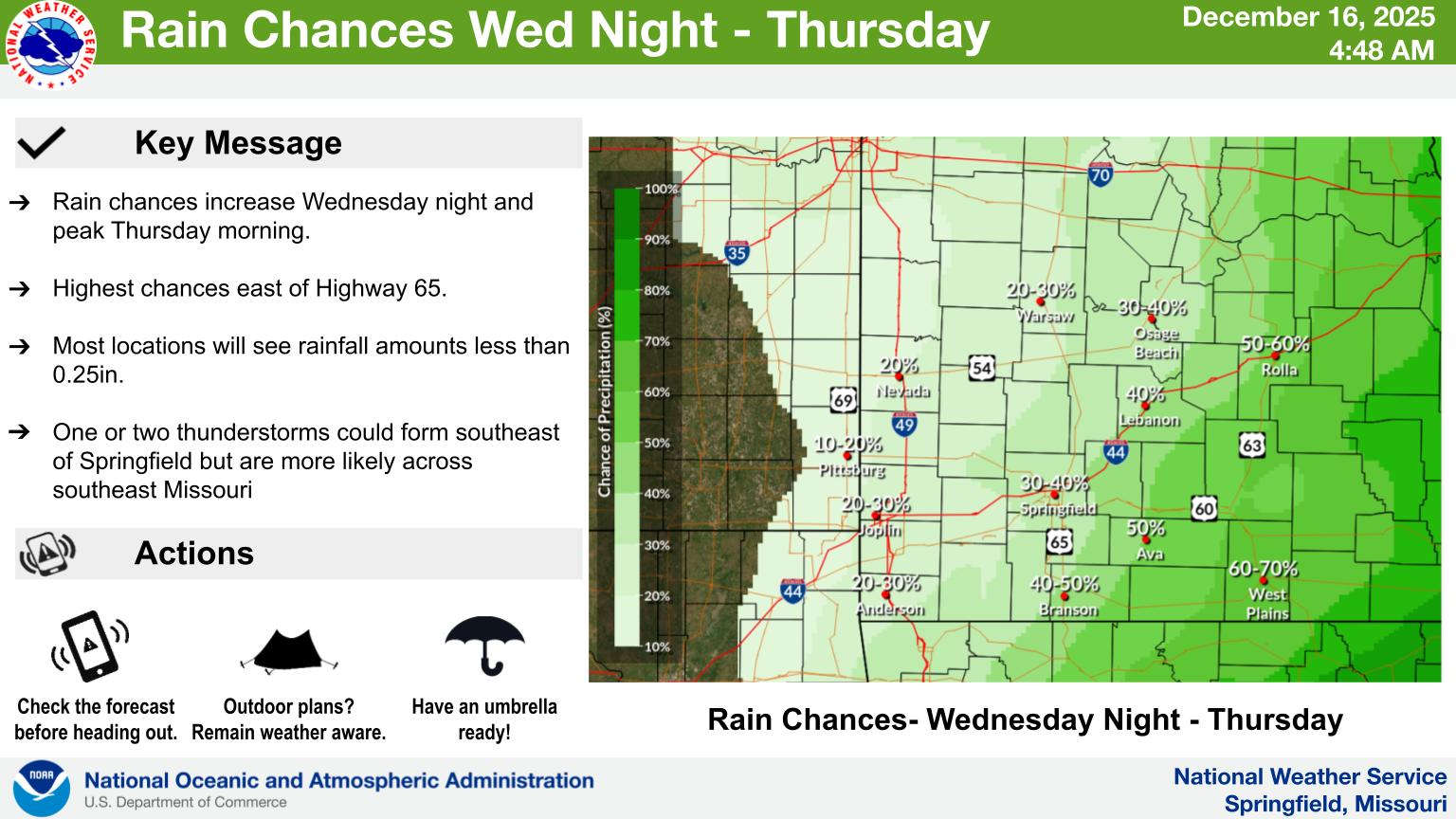 Rain Now in the Forecast for Later This Week
Rain Now in the Forecast for Later This Week
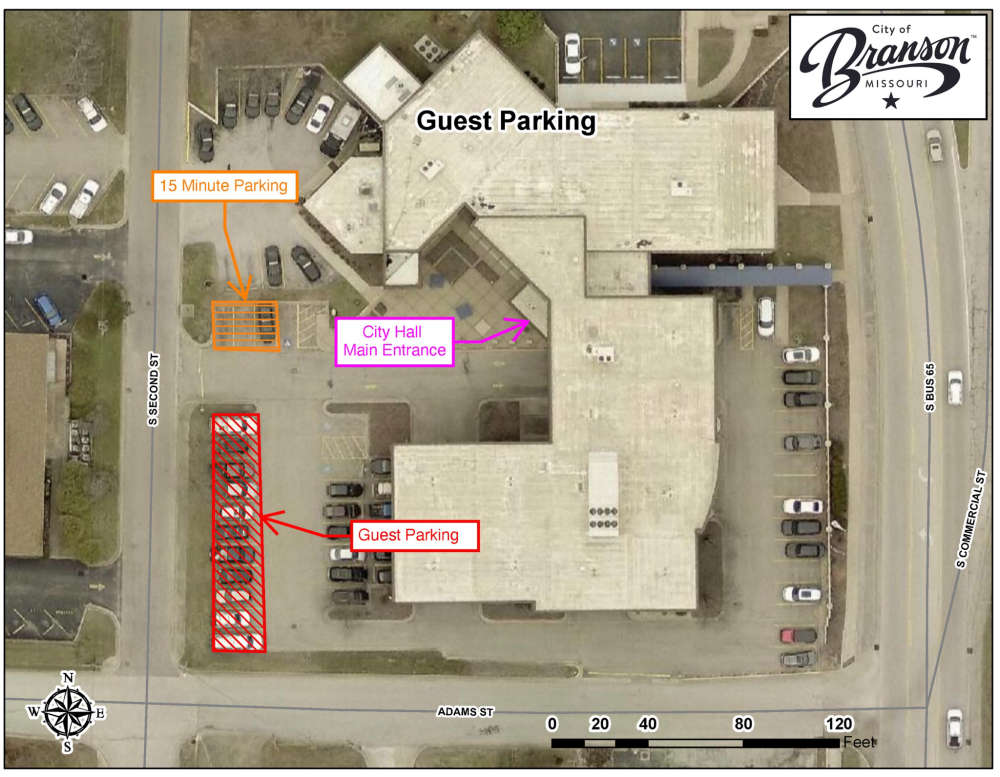 Second Floor Lobby Renovation Begins Today at Branson City Hall
Second Floor Lobby Renovation Begins Today at Branson City Hall
 Cold Conditions Challenge Firefighters Battling Blaze
Cold Conditions Challenge Firefighters Battling Blaze
 NWS Warns of Fire Danger
NWS Warns of Fire Danger